Review of Normal Values
- pH: 7.35–7.45
- Too low: Acidosis – reduces cardiac output, decreases sensitivity to catecholamines.
- Too high: Alkalosis – impairs oxygen delivery to tissues, disrupts neuro/muscular function.
- PaCO2: 35–45 mmHg (regulated by RESPIRATORY system)
- Increased: Respiratory acidosis or compensatory metabolic alkalosis.
- Decreased: Respiratory alkalosis or compensatory metabolic acidosis.
- HCO3: 22–26 mEq/L (regulated by KIDNEY – METABOLIC system)
Helpful Tips
Review the governing equation:
CO2 +H2O <–> H2CO3 <–> HCO3 + H+
Lungs <–> buffering <–> kidney
- Key equation:
CO2 + H2O ↔ H2CO3 ↔ HCO3 + H+- CO2 (carbon dioxide) combines with water (H2O) to form H2CO3 (carbonic acid), which breaks down into HCO3 (bicarbonate) and H+ (hydrogen ions, or acid).
- Lungs ↔ Buffering ↔ Kidneys
- Lungs: Regulate the CO2 levels, quickly adjusting the body’s pH by exhaling CO2.
- Buffering: The bicarbonate system (HCO3) neutralizes excess acids in the blood.
- Kidneys: More slowly regulate HCO3 and H+ levels, maintaining long-term pH balance.
Abnormal States
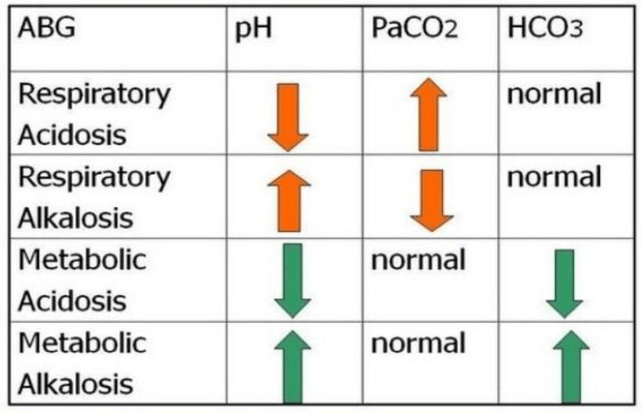
Respiratory Acidosis:
- pH < 7.35, PaCO2 > 45
- Causes: Pulmonary embolism, airway blockage, pneumothorax, severe COPD exacerbation.
- CO2 builds up due to hypoventilation (excess acid).
- Symptoms: CNS depression, fatigue, confusion.
Respiratory Alkalosis:
- pH > 7.45, PaCO2 < 35
- Hyperventilation leads to excess CO2 elimination (too little acid).
- Symptoms: Dizziness, lightheadedness, tingling.
- Causes: Anxiety, pain, fever, respiratory distress.
Metabolic Acidosis:
- pH < 7.35, HCO3 < 22
- Lack of bicarbonate (alkali).
- Causes: Renal failure, diabetic ketoacidosis, starvation, alcohol intoxication, sepsis, diarrhea in infants.
Metabolic Alkalosis:
- pH > 7.45, HCO3 > 26
- Too much base or not enough acid.
- Excess alkali: Overuse of antacids.
- Acid loss: Vomiting, gastric suction, diuretic use.
Uncompensated ABGs
Process for Interpreting an ABG
- Assess the pH: Is it above or below the normal range? (Alkalosis vs. Acidosis)
- Identify the Primary Cause: Determine if the imbalance is metabolic or respiratory by matching the pH disturbance with either CO2 or HCO3.
- Check for Compensation: Analyze the opposing value (CO2 or HCO3) to see if the body is trying to restore normal pH.
Examples
Example 1:
- pH: 7.2, CO2: 54, HCO3: 25
- pH Status: Low = Acidosis
- Primary Cause: CO2 is elevated (54), indicating excess acid = Respiratory cause
- Compensation Check: HCO3 is within normal limits (WNL) = No compensation
- Conclusion: Uncompensated respiratory acidosis
Example 2:
- pH: 7.82, CO2: 52, HCO3: 38
- pH Status: High = Alkalosis
- Primary Cause: HCO3 is elevated (38), indicating excess base = Metabolic cause
- Compensation Check: CO2 is also high (52), suggesting the body is retaining CO2 (acid) to compensate.
- Conclusion: Partially compensated metabolic alkalosis
Example 3:
- pH: 7.38, CO2: 20, HCO3: 20
- pH Status: Normal, but on the lower end = Compensated acidosis
- Primary Cause: HCO3 is low (20), meaning not enough base = Metabolic cause
- Compensation Check: CO2 is low (20), indicating the body is blowing off CO2 to balance the pH.
- Conclusion: Fully compensated metabolic acidosis
Example 4:
- pH: 7.39, CO2: 51, HCO3: 37
- pH Status: Normal, but leaning towards low = Compensated acidosis
- Primary Cause: CO2 is elevated (51), indicating excess acid = Respiratory cause
- Compensation Check: HCO3 is high (37), suggesting the kidneys are increasing bicarbonate to neutralize the acidity.
- Conclusion: Fully compensated respiratory acidosis