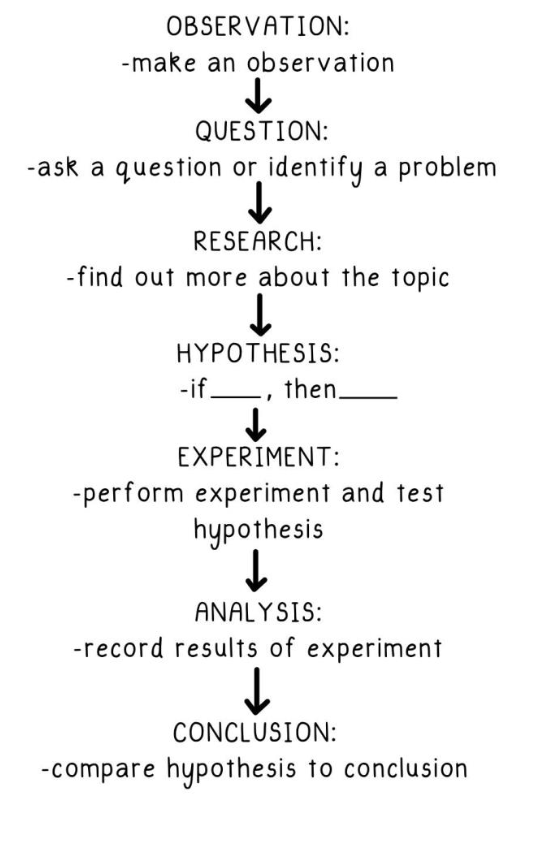
Properties of Atoms
Atoms
- The basic building blocks of matter, composed of three subatomic particles: protons, neutrons, and electrons.
- Structure:
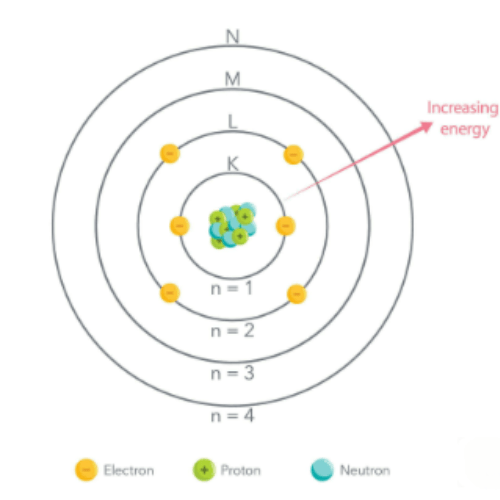
- Nucleus: Central core containing protons (positively charged) and neutrons (neutral).
- Electron Cloud: Surrounds the nucleus and holds electrons (negatively charged), arranged in shells or energy levels.
- Energy Levels: Electrons further from the nucleus have higher energy, and moving an electron away requires energy due to the attraction between negative electrons and the positive nucleus.
- Structure:
Key Concepts
- Isotopes: Variants of an element with different numbers of neutrons.
- Atomic Mass: Calculated as the total number of protons and neutrons in the nucleus.
- Atomic Number: Defined by the number of protons in an atom.
- Ions: Atoms with an electrical charge.
- Cations: Positively charged ions.
- Anions: Negatively charged ions.
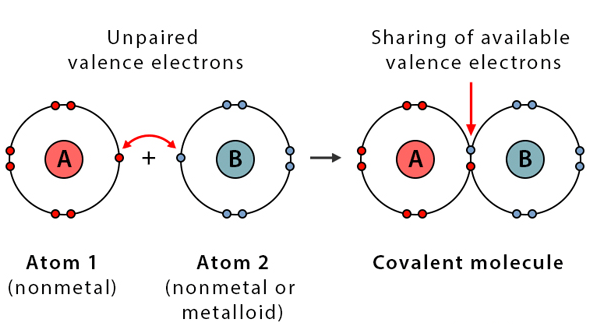
- Valence Electrons: Electrons in the outermost shell; atoms are most stable when their valence shell is full.
- Electron Gain: Leads to a negative charge.
- Electron Loss: Leads to a positive charge.
Electron Organization
- Shell: The primary pathway electrons follow around the nucleus.
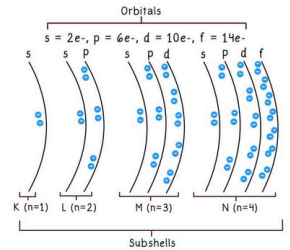
- Subshell: Divisions within a shell.
- Orbital: Specific regions in subshells where electrons are likely to be found (e.g., s, p, d, f).
- Electron Capacity Formula: Maximum electrons in an energy level = 2n² (where n is the energy level number). For example, the 4th energy level can hold 32 electrons (2 × 4² = 32).
Types of Chemical Bonds
Covalent Bonds
- Formed when two atoms share electrons equally to achieve stability.
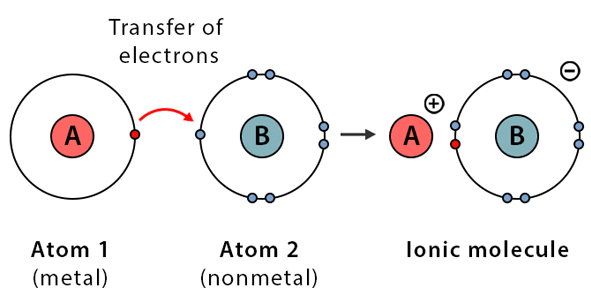
Ionic Bonds
- Created when electrons are transferred from one atom to another, resulting in one atom predominantly holding the electrons to achieve stability.
Physical Properties of Matter
Physical Properties
- Characteristics that can alter the state of a substance without changing its identity.
- Example: Steam produced from boiling water retains the same molecular structure as liquid water.
Key Measures
- Mass: Reflects the number of molecules in a substance.
- Volume: Indicates the amount of space occupied by the molecules in a substance.
- Density: The ratio of mass to volume, describing how compact a substance is.
States of Matter
- Matter exists in four distinct phases (solid, liquid, gas, plasma).
- Phase Determinants:
- Temperature: Raising temperature increases molecular motion, pushing particles apart.
- Pressure: Increasing pressure brings particles closer together.
- Phase Determinants:
Kinetic Molecular Theory
- Describes how molecular motion varies with heat energy; motion increases when heat is added and decreases when heat is removed.

Water
- Polarity: Water molecules have a negatively charged oxygen side and positively charged hydrogen sides.
- Cohesion: Water molecules cling to one another, creating surface tension.
- Adhesion: Water molecules adhere to other particles or surfaces.
- Universal Solvent: Water dissolves many substances, making it essential in various chemical processes.
Solvents and Solutes
Definitions
- Solute: The substance being dissolved.
- Solvent: The medium dissolving the solute.
- Solution: A homogeneous mixture formed when solutes dissolve in solvents.
Dilution
- Involves adding more solvent to decrease the concentration of solute.
Concentration Measures
- Molarity (mol/L): Moles of solute per liter of solution; depends on temperature.
- Mole Fraction (mol/mol): Moles of solute divided by total moles in the mixture.
- Molality (mol/kg): Moles of solute per kilogram of solvent.
- Mass Percentage (g/g): Ratio of solute mass to solution mass.
- Parts Per Thousand (PPT): Grams of solute per kilogram of solution.
- Parts Per Million (PPM): Milligrams of solute per kilogram of solution.
- Parts Per Billion (PPB): Micrograms of solute per kilogram of solution.
Diffusion and Osmosis
- Diffusion: Movement of substances from areas of higher concentration to lower concentration (e.g., gas exchange in lung capillaries).
- Osmosis: Water movement across a membrane, flowing from higher to lower water concentration.
- Active Transport: Energy is required to move substances from areas of lower to higher concentration.
Chemical Reactions
Catalysts (enzymes): reduce the activation energy needed for a reaction, accelerate the process, and can be utilized repeatedly.
Synthesis: the process of combining components to form a more complex substance or structure.
A + B >- AB
Decomposition: a chemical reaction in which a compound acts as the reactant and is broken down into simpler products.
AB – > A + B
Single replacement: a reaction where one element within a compound is replaced by a different element, resulting in the formation of a new compound and a separate element as the products.
AB + C >- AC + B
Double replacement: a reaction in which two compounds swap ions, resulting in the creation of two entirely new compounds.
AB + CD – > AC + BD
Combustion: a process where a fuel combines with oxygen gas, producing carbon dioxide and water as the end products.
C× + Oy – > CO2 + H20
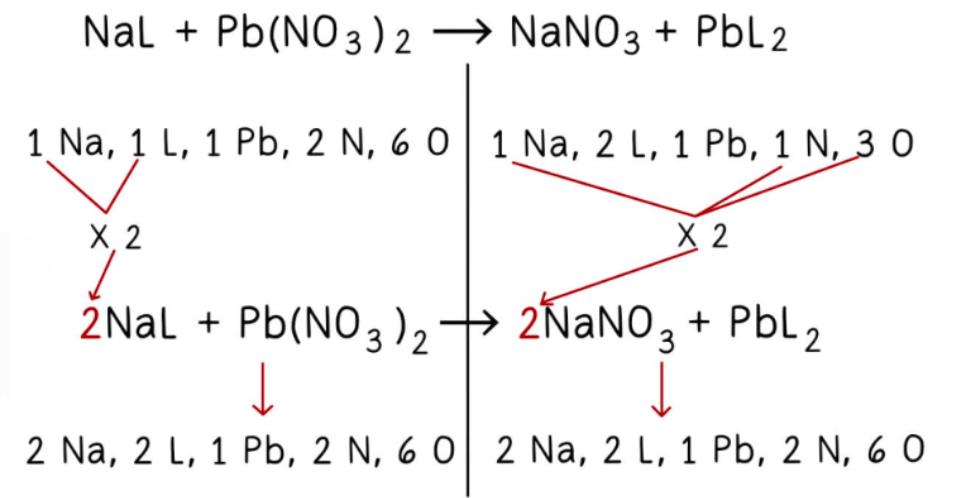
Balancing Chemical Reactions
- Tally the number of atoms for each element on both sides of the equation.
- Apply the smallest possible coefficient to equalize the number of atoms for each element on both sides (consider atoms grouped in compounds).
- The equation is now balanced.
Moles
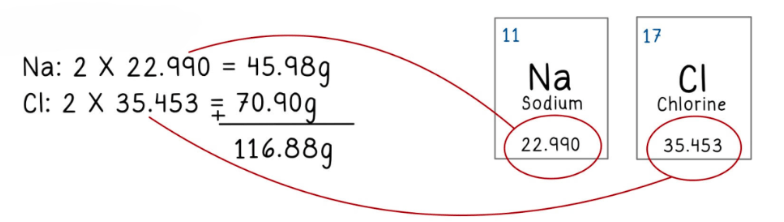
- One mole corresponds to the atomic mass of a substance in grams. For example, carbon has an atomic mass of 12 g, so 12 g of carbon equals one mole.
- The number of moles for a substance can be determined using the atomic masses listed in the periodic table.
- For compounds, the atomic masses of individual elements are summed to calculate the total mass.
What is the mass in grams for 2 moles of NaCl?
Acid and Bases
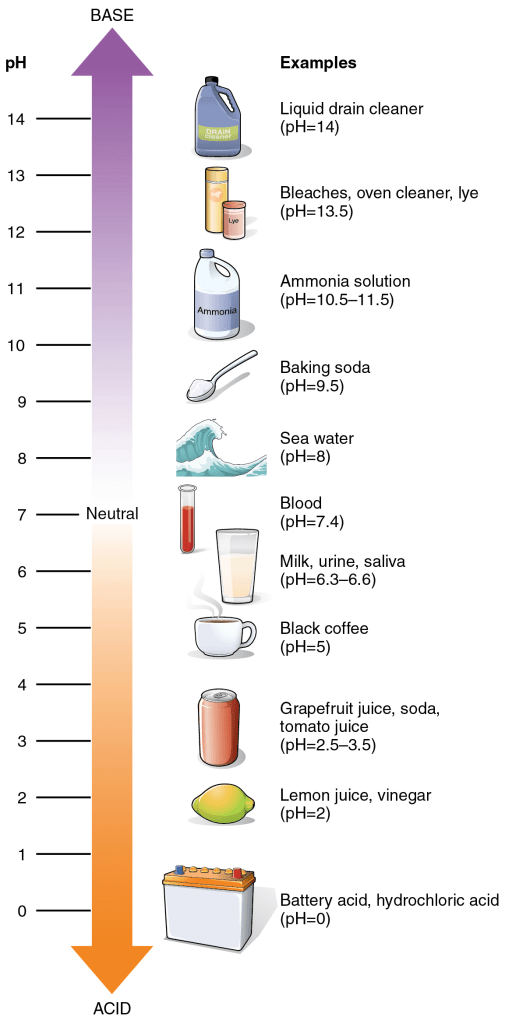
- pH: A scale that quantifies the concentration of hydrogen ions in a solution, ranging from 0 to 14.
- Acids (hydrogen ion donors): Typically have a sour taste, a pH below 7, and a higher concentration of hydrogen ions than hydroxide ions.
- Introducing an acid to a solution decreases its pH.
- Bases (hydrogen ion acceptors): Generally have a bitter taste, a pH above 7, and a higher concentration of hydroxide ions than hydrogen ions.
- Adding a base to a solution increases its pH.
- Neutral: A solution with a pH of exactly 7, indicating it is neither acidic nor basic.
- Buffer: A solution that stabilizes pH by minimizing shifts when acids or bases are introduced. Buffers help maintain pH balance in biological systems.
- Buffers absorb excess H+ or OH- ions to prevent significant pH changes.
- pOH: A measure of hydroxide ion concentration in a solution. It is inversely related to pH— as pH increases, pOH decreases.
- Formula: pH + pOH = 14
- pH = -log[H+]
- pOH = -log[OH-]
Neutralization
Water formation from hydrogen and hydroxide ions:
- When an acid reacts with a base, the hydrogen ions (H+) from the acid bond with the hydroxide ions (OH-) from the base, resulting in the formation of water.
- If the quantities of acid and base are equal, the mixture reaches a neutral state.
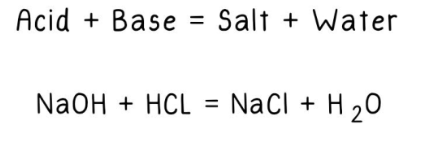
Scientific Reasoning
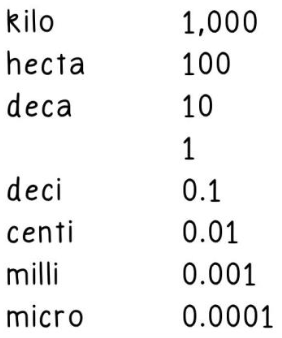
Frequently used measurement prefixes:
The metric system is employed for measuring and recording data.
Measurement tools:
- Length: ruler, meterstick
- Solid volume: determined by multiplying length, width, and height
- Liquid volume: measured with a volumetric flask, pipette, or graduated cylinder
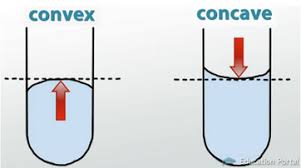
- Liquids create a meniscus: a curve at the liquid’s surface due to the container’s walls.
- Concave: upward curve; Convex: downward curve
- To measure liquid, read the value at the line level with the meniscus’ center.
- Independent variable: the factor that is intentionally altered or adjusted in an experiment, typically represented on the X-axis of a graph.
- Dependent variable: the outcome or result that is observed and measured after the independent variable is modified, usually depicted on the Y-axis of a graph.
- Controlled variables: all the factors that are kept constant and unchanging to ensure the experiment’s accuracy and validity.
Scientific Method